For 30 years, researchers have struggled to determine which immune responses best foil HIV, information that has guided the design of AIDS vaccines and other prevention approaches. Now, a research team has shown that a lab-made molecule that mimics an antibody from our immune system may have more protective power than anything the body produces, keeping four monkeys free of HIV infection despite injection of large doses of the virus.
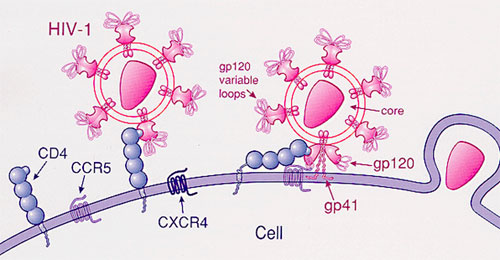
Intensive hunts are under way for natural HIV antibodies that can stop—or “neutralize”—the many variants of the constantly mutating AIDS virus. Researchers have recently found several dozen broadly neutralizing antibodies (bNAbs) that are highly potent and work at low doses. But viral immunologist Michael Farzan of the Scripps Research Institute in Jupiter, Florida, and 33 co-workers have recently taken a different strategy, building a novel molecule based on our knowledge of how HIV infects cells. HIV infects white blood cells by sequentially attaching to two receptors on their surfaces. First, HIV’s own surface protein, gp120, docks on the cell’s CD4 receptor. This attachment twists gp120 such that it exposes a region on the virus that can attach to the second cellular receptor, CCR5. The new construct combines a piece of CD4 with a smidgen of CCR5 and attaches both receptors to a piece of an antibody. In essence, the AIDS virus locks onto the construct, dubbed eCD4-Ig, as though it were attaching to a cell and thus is neutralized.
In test-tube experiments, eCD4-Ig outperformed all known natural HIV antibodies at stopping the virus from infecting cells, Farzan’s team reports in this week’s issue of Nature. To test how it works in animals, they then put a gene for eCD4-Ig into a harmless virus and infected four monkeys; the virus forces the monkey’s cells to mass produce the construct. When they “challenged” these monkeys and four controls with successively higher doses of an AIDS virus for up to 34 weeks, none of the animals that received eCD4-Ig became infected, whereas all of the untreated ones did.
The new study ups the ante on a similar gene therapy approach with natural antibodies that 6 years ago showed promise in monkey experiments, says an accompanying Nature editorial by AIDS vaccine researcher Nancy Haigwood of Oregon Health & Science University in Beaverton. “I am a huge fan of this paper,” Haigwood says. “It’s really very creative and a breakthrough as far as I am concerned.” Pediatrician Philip Johnson of the Children’s Hospital of Philadelphia in Pennsylvania, whose lab in 2009 showed success with a gene therapy that delivers an HIV bNAb, adds that eCD4-Ig “is a beautiful thing.”
Building on work by Johnson’s group, Farzan’s team stitched the gene for eCD4-Ig into an adeno-associated virus (AAV) that is harmless to humans. Those viruses, injected into monkey muscles, continued to produce eCD4-Ig for the 40 weeks of the experiment. “Everyone expects with AAV that this can go on forever,” Farzan says. The animals had no detectable immune response against the eCD4-Ig, presumably because it is so similar to pieces of their own cells.
Not everyone is convinced that eCD4-Ig will ultimately work better than natural HIV antibodies. Virologist David Baltimore, a Nobel laureate based at the California Institute of Technology in Pasadena, is working with a group developing its own AAV gene therapy that delivers an HIV bNAb. He describes the eCD4-Ig chimera and the paper as “impressive” and says he welcomes this new approach. But Baltimore, who like Johnson has already moved into early phase human trials with his gene therapy, notes that the new work offers only test-tube and animal data. “It’s perhaps a better construct than the antibodies we’ve been using, but it’s a matter of how it plays out in human trials,” Baltimore says. “I don’t think it’s easy to tell how that will happen.”
Johnson agrees that eCD4-Ig may not work as well as bNAbs in humans, but also says the natural antibodies, even if they have less potency and breadth, may be powerful enough to stop HIV. “How good is good enough?” Johnson asks. “Nobody has a clue about that. The only way you would know really is to do a bake-off in a human trial.”
Farzan says in theory at least, it will be harder for the virus to mutate its way around eCD4-Ig than a bNAb, because HIV needs to bind to CD4 and CCR5. Whether any of these gene therapies will prove safe and practical remains to be seen. Farzan, for his part, has more experiments planned before moving into humans. “We need to do a lot more monkey studies to see if there’s anything weird,” he says.
sciencemag

